China is a country with a high incidence of gastric cancer. Both the incidence rate and mortality of gastric cancer rank second in malignant tumors with about 400,000 new cases and 350,000 deaths each year. Both new cases and deaths account for more than 40% of the world’s cases. Therefore, reducing the incidence rate and mortality of gastric cancer in China has become an urgent task. However, 90% of gastric cancer found in China is in the advanced stage, and the early detection rate is lower than 10%, far lower than that of Japan and South Korea. The prognosis of gastric cancer is closely related to the diagnosis and treatment period. The 5-year survival rate of patients with advanced gastric cancer is still lower than 30% even after receiving comprehensive treatment mainly based on surgery, and the quality of life is low, which brings heavy burden to patients and their families. The 5-year survival rate of early gastric cancer after endoscopic treatment can exceed 90%, and even be cured. Therefore, the realization of early screening, early diagnosis and treatment of gastric cancer is an effective means to solve the current severe situation of high incidence rate and mortality of gastric cancer in China.
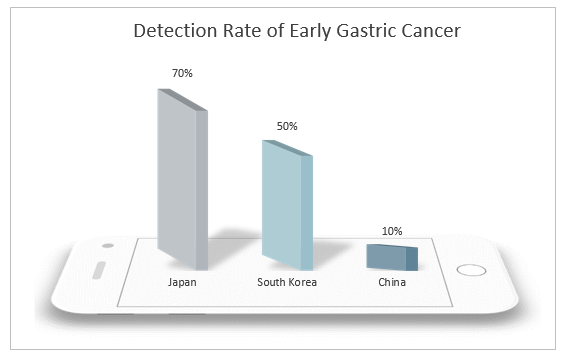
The incidence rate of gastric cancer in natural population in China is low, about 31.28/100,000 people. At present, there is no simple and effective diagnostic method for general population detection. Although gastroscopy and biopsy are the gold standard for the diagnosis of gastric cancer, as an invasive means, the equipment and doctor levels are high, and it is expensive and painful, and therefore has poor patient acceptance, making it more difficult to conduct large-scale gastric cancer screening. Thus, the non-invasive diagnostic methods and risk factors such as age and sex shall be considered to screen the high-risk population of gastric cancer and then a comprehensive endoscopic detection shall be conducted, which is deemed as a more feasible screening means.
Pepsinogen (PG),PG) is an inactive precursor of pepsin including PGI and PGII subtypes and a good indicator of exocrine function of gastric body and antrum mucosa and can be call “Serological Biopsy”. With the development of gastric disease, PGI in serum first increases and then decreases, and PGII increases and then maintains a high level. Thus, the abnormality of PGI, PGII, and PGI/PGII ratio will indicate different gastric diseases. Therefore, PG is not only an important indicator for early screening of gastric cancer but also a means for early screening treatment and monitoring of superficial gastritis, erosive gastritis, gastric ulcer, duodenal ulcer, atrophic gastritis, and other gastric diseases.
In 2017, the National Clinical Research Center for Digestive Diseases (Shanghai) established a new gastric cancer screening scoring system based on clinical research (Table 1). According to the score, the target population of gastric cancer screening can be divided into three grades: High risk population of gastric cancer (17-23 points) with an extremely high possibility of gastric cancer; middle risk population of gastric cancer (12-16 points) with a certain possibility of gastric cancer; low risk population of gastric cancer (0-11 points) with an average risk of gastric cancer.
Table 1 A New Scoring System for Gastric Cancer Screening
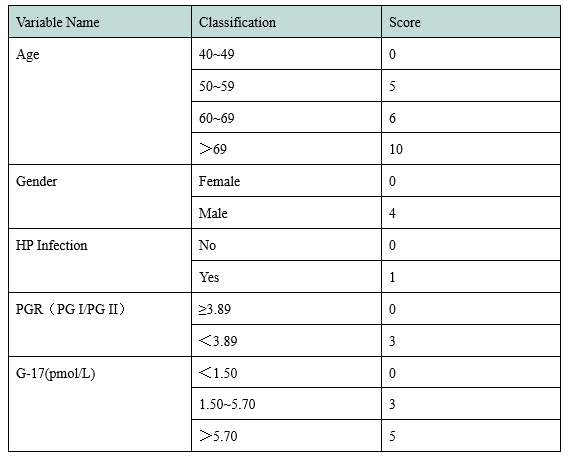
In the new gastric cancer screening and scoring system, age, gender, PGI/PGII and G-17 are all given high scores. Henderson launched PGI and PGII antigen testing materials, and will later launch G-17, HP antigen and other raw materials to assist in the research and development of gastric function testing kit.
Product Information
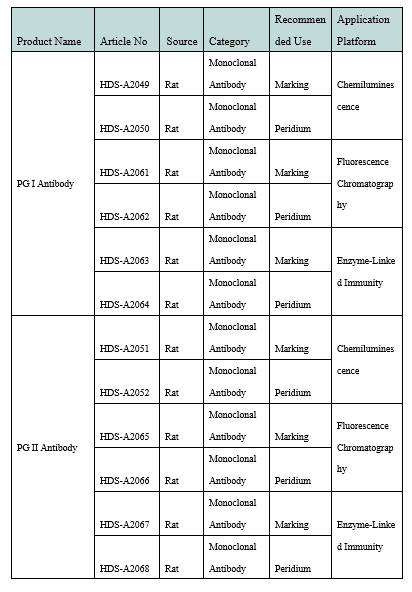
Product Performance
【PGI】
On the chemiluminescence platform, the linear correlation of PGI paired monoclonal antibody in the range of 0-325ng/mL was above 97.9%; the PGI paired monoclonal antibody was used to benchmark the kit of the well-known manufacturer in the field of gastric function. The serum agreement rate R2 was>98%.
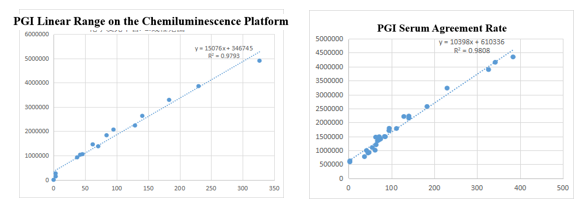
On the enzyme-linked immunity: The linear correlation of PGI paired monoclonal antibody in the range of 0-210ng/mL is more than 99%; the PGI paired monoclonal antibody was used to benchmark the kit of the well-known manufacturer in the field of gastric function. The serum agreement rate was R2>97%.
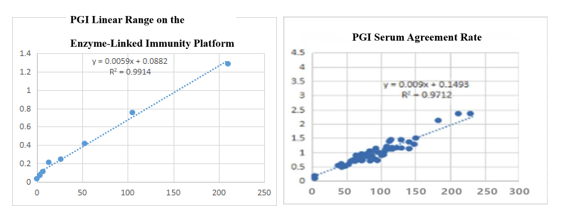
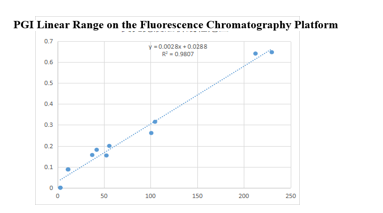
On the fluorescence chromatography platform: the linear correlation of PGI paired monoclonal antibody reached more than 98% within the range of 3-229ng/mL
【PGII】
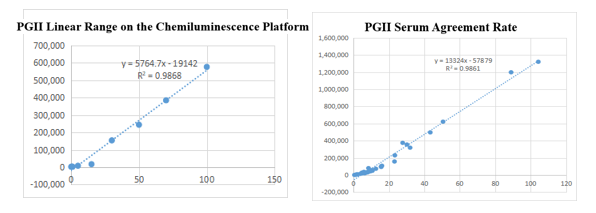
On the chemiluminescence platform, the linear correlation of PGII paired monoclonal antibody in the range of 0-100ng/mL was above 98.6%; the PGII paired monoclonal antibody was used to benchmark the kit of the well-known manufacturer in the field of gastric function. The serum agreement rate was R2 >98%.
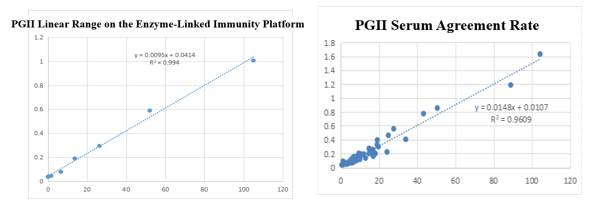
On the enzyme-linked immunity: The linear correlation of PGII paired monoclonal antibody in the range of 0-105ng/mL is more than 99.4%; the PGII paired monoclonal antibody was used to benchmark the kit of the well-known manufacturer in the field of gastric function. The serum agreement rate was R2>96%.
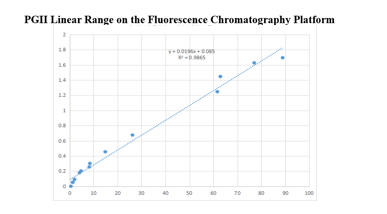
On the fluorescence chromatography platform: the linear correlation of PGII paired monoclonal antibody reached more than 98% within the range of 0-100ng/mL
Reference
Expert Consensus on Early Gastric Cancer Screening Process in China (2017 Shanghai)
[Company Background]
Qingdao Henderson Biotechnology Co., Ltd, established in 2016, serves as a professional supplier of core raw materials for in vitro diagnostic reagents and is committed to providing high-quality raw materials for global in vitro diagnostic reagent production enterprises, scientific research institutions and other fields.
The company provides the following staple products: antigens, antibodies, enzymes, etc., widely used in the detection of drug, respiratory infectious diseases (A, B, COVID-19 antigen, COVID-19 antibody, COVID-19 neutralizing antibody, syncytial virus, etc.), other infectious diseases (hepatitis, AIDS, tuberculosis, etc.), markers for tumor, inflammation, myocardial markers, animal diseases detection (dog, cat diseases) and other fields.
The company, after years of development, has established several specialized laboratories and SPF experimental animal bases, a comprehensive and systematic R&D verification platform and formed a professional technical service team to provide personalized customized services and one-stop solutions.
Qingdao Henderson Biotechnology Co., Ltd is willing to build a renowned product brand for all customers with high-quality products and professional services to keep a foothold in the market with its sound competitiveness.

Limited space only allows the introduction of major products. You are welcomed to contact Qingdao Henderson Biotechnology Co., Ltd and acquire trial samples if you need more information.
Qingdao Henderson Biotechnology Co., Ltd.
Official Website: /
Tel: 0532-84670782
E-mail: info@qdhenderson.com